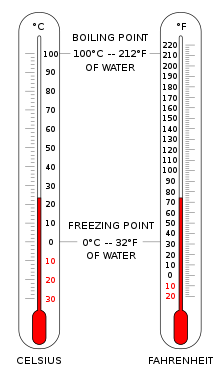
Fahrenheit is the temperature scale proposed in 1724 by, and named after, the German physicist Daniel Gabriel Fahrenheit (1686–1736). Within this scale, the freezing of water into ice is defined at 32 degrees, while the boiling point of water is defined to be 212 degrees. The temperature scale was replaced by the Celsius scale in most countries during the mid to late 20th century, but it remains the official scale of the United States, Cayman Islands and Belize.
Fahrenheit is the temperature scale proposed in 1724 by, and named after, the German physicist Daniel Gabriel Fahrenheit (1686–1736). Within this scale, the freezing of water into ice is defined at 32 degrees, while the boiling point of water is defined to be 212 degrees. The temperature scale was replaced by the Celsius scale in most countries during the mid to late 20th century, but it remains the official scale of the United States, Cayman Islands and Belize. |
| Credit: uoregon.edu |